A recent study published in the journal Nature Materials titled High stability and luminescence efficiency in donor–acceptor neutral radicals not following the Aufbau principle (Nature Materials, 2019, 18, 977-984, DOI: 10.1038/s41563-019-0433-1) claims that researchers have provided a simple molecular-design strategy for stable, highly luminescent radicals with the non-Aufbau electronic structures , and fabricated a near-infrared organic light emitting diode (OLEDs) with an external quantum efficiency (EQE) as high as 5.3 % based on such a radical emitter, which demonstrates the application prospects of luminescent radicals in organic optoelectronics.

The report was jointly published by Professor Feng Li’s group from the State Key Laboratory of Supramolecular Structure and Materials at Jilin University, Professor Jean-Luc Brédas’s group from the Georgia Institute of Technology and Professor Richard H. Friend’s group from the Cavendish Laboratory at the University of Cambridge, with Jilin University the first affiliation.
Co-corresponding authors: Prof. Feng Li (Jilin University), Prof. Jean-Luc Brédas (Georgia Institute of Technology) and Prof. Ricard H. Friend (University of Cambridge)
Co-first authors: Mr. Haoqing Guo (Jiin University), Associate Research Fellow Qiming Peng (Jilin University/ Nanjing University of Technology), Dr. Xiankai Chen (Georgia Institute of Technology)
First affiliation: the State Key Laboratory of Supramolecular Structure and Materials at Jilin University
Being of high contrast, ultra-thinness and flexibility, organic light emitting diodes (OLEDs) have a huge market value and great potential in the field of display and lighting, and have been used in devices such as mobile phones and televisions. As up to 75% of the excitons in OLEDs are triplet excitons, which do not emit light due to spin-forbidden transitions, scientists have been hard at work for the last three decades trying to exploit triplet excitons to arrive at 100% Internal Quantum Efficiency (IQE). They normally use phosphorescence and thermally activated delayed fluorescence (TADF) to utilize both singlet and triplet excitons generated in OLEDs in order to achieve 100% IQE.
Working in OLEDs field for over 20 years, after understanding the nature of the spin-forbidden transition in triplet excitons, Prof. Feng Li proposed a new electroluminescent principle of OLEDs, doublet emission. Luminescent radical materials were employed as the emitters which only generate doublet excitons in the light-emitting region of OLEDs, and the transition of doublet excitons is spin-allowed, thus, the theoretical upper limit of IQE can be improved to 100%, and the problem of triplet exciton utilizing can be circumvented. Based on this principle, Professor Li's research team first applied a luminescent radical molecule, TTM-1Cz, in the light-emitting layer of OLEDs in 2015 to produce a red-emitting device, and confirmed that its emission comes from the transition of doublet excitons (Angew. Chem., Int. Ed., 2015, 54, 7091-7095). In the following research, the team continued to improve the performance of luminescent radical materials and devices, and proved that 100% formation ratio of doublet excitons of OLEDs based on radical luminescent materials can be achieved (ACS Appl. Mater. Interfaces, 2016, 8, 35472-35478; Chem. Mater., 2017, 29, 6733-6739; J. Phys. Chem. Lett., 2018, 9, 6644-6648). Furthermore, the team designed and synthesized a novel kind of stable luminescent radicals, biphenylmethyl radicals, expanding the material system of luminescent radicals (Angew. Chem., Int. Ed., 2018, 57, 2869-2873). In 2018, the research team acquired two highly efficient radicals, TTM-3NCz and TTM-3PCz, and the photoluminescence efficiencies of their doped films reached 90% and 61%, respectively. The maximum external quantum efficiency (EQE) of OLEDs based on the two radical molecules reached 27% and 17%, respectively. (Nature, 2018, 563, 536-540).
In order to further improve the stability of luminescent radicals, based on previous research results, Professor Li and his team connected TTM or PTM radicals as acceptors and PDCz, PPTA and 3NCz carbazole derivatives as donors to obtain a series of donor–acceptor radicals, such as PTM-3NCz, PTM-PDCz and TTM-PPTA (as shown in Figure 2). The donor-acceptor property of this type of radicals endows them with non-Aufbau electronic structures, which greatly improves the stability of the luminescent radicals. Compared to TTM radicals, these non-Aufbau structured donor-acceptor radicals is 2-4 orders of magnitude more stable.
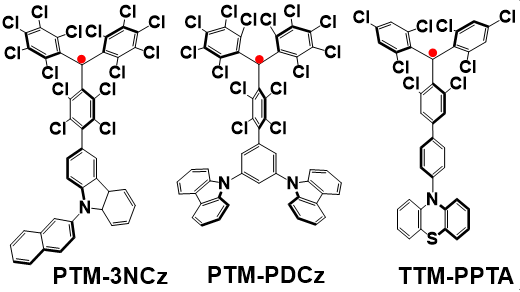
Figure 2 Molecular Structures of Donor-Acceptor Radicals
First proposed by Niels Bohr in 1923, the Aufbau principle states that when electrons fill atomic (or molecular) orbitals, they will fill in pairs and start from the lowest available energy levels before occupying higher ones (as shown in figure 3a). The non-Aufbau principle refers to the scenario in which higher-energy orbitals of an atom (or molecule) have been filled with paired electrons while the lower-energy ones are still available (as shown in figure 3b). Professor Li and his team carried out a series of experiments, including ultraviolet photoelectron spectroscopy (UPS), cyclic voltammetry (CV) and absorption and emission spectra, to confirm that the donor-acceptor radicals exhibit an non-Aufbau electronic structure. To further explore this particular electronic structure, the team collaborated with Prof. Jean-Luc Brédas from the Georgia Institute of Technology to perform a whole string of computations in quantum chemistry based on density functional theory (DFT) and multi-configuration self-consistent field method (MCSCF). The computations not only confirmed the non-Aufbau electronic structure in donor-acceptor radicals, but also analysed conditions for such non-Aufbau electronic structures to form in view of orbital hybridization.
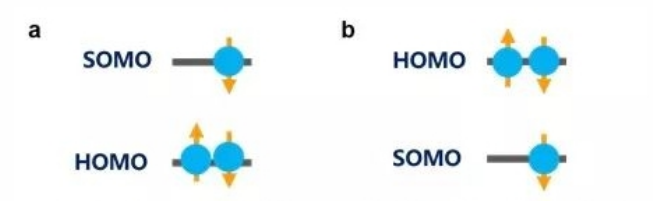
Figure 3 a) Ground State Electronic Structure of a Molecule that Follows the Aufbau Principle
Figure 3 b) Ground State Electronic Structure of a Molecule that Does not Follow the Aufbau Principle
With the support of the the Talents Cultivation Project of Jilin University, the team cooperated with Prof. Richard H. Friend’s research group at the University of Cambridge to further explore the OLED application of this kind of radicals. By using PTM-3NCz as the emitter, a near-infrared solution-proceesed OLED could reach an maximum EQE of 5.3%, among the highest values ever achieved for pure near-infrared OLEDs.
This research received the financial supports from National Natural Science Foundation of China (91833304, 51673080, 11804156), the Ministry of Science and Technology’s Key Research and Development Program and the "973" Program (2016YFB0401001, 2015CB655003), Jilin University Talents Cultivation Project and high-level scientific and technological innovation team construction projects.
About the author: Feng Li, professor and doctoral supervisor at the State Key Laboratory of Supramolecular Structures and Materials at Jilin University. In recent years, he has focused his research on high-efficiency organic luminescent materials and devices based on novel emission mechanisms, and has published his research work in top international journals including Nature, Nat. Mater., Angew. Chem. Int. Ed. and Adv. Mater. His research about the luminescent radial materials and devices with doublet excitons has obtained Chinese and American patents (ZL201410018393.9, ZL201410639989.0, US9935271B2), with independent intellectual property rights.


